CATEGORIES
فئات
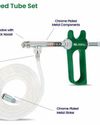
Blissfill-Calibrated syringe pipettes for repetitive laboratory dosing as per ISO 8655-9
Blissfill syringe pipettes provide laboratories with an ergonomic, self-refilling solution for repetitive dosing, delivering precise volumes from 0.05ml to 10ml. With ISO 8655-9 compliance and an included calibration certificate, Blissfill ensures accuracy and ease of use for high-demand lab applications

Ensuring pharma compliance with testo data measurement technology
Comprehensive data measurement tools address regulatory demands and secure quality in pharma

Waters launches software for lab-centric business intelligence enabling audit readiness
waters_connect Data Intelligence software provides laboratories with comprehensive business intelligence on their chromatographic data throughout the product development lifecycle, enabling real-time audit readiness and query response, while reducing time spent on audit preparation

Ensuring clean room integrity with Prime Clean Reset High-Speed Doors
Designed with precision to meet the stringent requirements of controlled environments, Prime Clean Reset is the epitome of performance and reliability, ensuring that your clean room operations consistently meet the highest standards of regulatory compliance and product integrity

Optimising efficiency with Remote Laboratory Solutions
LabWare's comprehensive suite of software solutions designed specifically for laboratories offers reliable Remote Laboratory Solutions to help your business thrive in the current climate

Ensuring water quality with the 6000TOCi: The ultimate solution for continuous TOC measurement
The 6000TOCi Sensor is a comprehensive solution that brings together continuous monitoring, rapid response, reliability, and compliance

Enhancing pharmaceutical analysis in critical areas
Dheeraj Handique, Manager GC/GCMS Product Marketing, Shimadzu Analytical (India) highlights the benefits of GCMS-TQ8050 NX with HS-20 NX trap for genotoxic impurities, nitrosamines, and, extractables and leachables

Technical innovations for Annex 1
To meet the stringent EU GMP Annex 1 requirements, Optima presents innovative solutions to improve product and patient safety in pharmaceutical manufacturing. From minimising glove interventions and optimising First Air flow to advanced automation for integrity testing, this article explores how Optima's technologies support compliance in aseptic fill-and-finish processes

JRS Pharma - The Global MCC Manufacturing Network
JRS Pharma is one of the largest manufacturers of MCC across the globe. The know-how is based on more than 25 years of experience in development of own processes

Mack Pharmatech is the first to launch PLC-based environmental chambers in India
Manoj Choudhari, Managing Director, Mack Pharmatech shares insights on his company's pioneering expand globally and enhance regulatory compliance

Delivering on GLP-1 demand: A combination of device offerings and supply strategy
Manuela Giacon, Product Manager, Pen Injectors, Stevanato Group & Josh Gordon, Product Manager, Auto-Injectors, Stevanato Group explain why auto-injectors and pen injectors will remain at the fulcrum of GLP-1 delivery

We aim to strengthen our presence in emerging pharma hubs across Tier 2 and Tier 3 cities.
Dr Kiran Badgujar, Founder & Director, Aureole Pharmatech discusses his company's strategic initiatives to strengthen its presence in emerging pharma hubs, innovate through sustainable, customisable lab solutions, future technology investments and more, in an interview with Express Pharma

Securing the pharma supply chain through traceability, transparency and technology
Traceability has long been a challenge for the pharma supply chain. Yet, it is key to ensure good distribution practices and overcome the constant threat of counterfeiting drugs. Industry experts analyse the challenges, and strategise to safeguard the pharma supply chain

Access to Medicine Index flags slowing momentum, especially in LMICS
The Access to Medicine Foundation, has recently released an Index ranking and evaluating 20 of the world's leading pharmaceutical companies according to their efforts to expand access to their products for people living in low-and middle-income countries. The 2024 Index highlights that, despite progress in several areas, the overall momentum has slowed compared to the previous Index

Maypharm is actively exploring opportunities for partnerships and acquisitions
Sameer Kolhe, President, Maypharm, divulges how his company is leveraging technology-driven products, tailored business models, and niche market strategies to drive growth and address unmet medical needs, in an interview with Lakshmipriya Nair

Romaco at CPHI & PMEC India 2024: Processing and more
Romaco will be taking advantage of this year's CPHI & PMEC India to show its complete processing portfolio for granulating, tableting and coating pharma solids. The pharma machinery manufacturer also provides numerous primary, secondary and final packaging solutions for a wide variety of products

SRICO: A trusted partner to enhance scientific research
Since 2011, SRICO has been a trusted provider of high-quality scientific instruments to diverse sectors, including pharmaceuticals, life sciences, and environmental science. With dedicated service centers and expert support teams across India, SRICO ensures tailored, compliant, and reliable solutions for research and production needs

Think global, act local - Romaco's approach to the Indian pharma market
The Indian pharmaceutical market is dynamic and increasingly international, which is reflected in trade fairs such as CPHI & PMEC India. Jens Torkel, MD, Romaco Group, a leading international supplier of machines and integrated system solutions for the development, manufacture and packaging of pharmaceuticals, and Sanjeev A. Nimkar, MD, Romaco India, Romaco's Sales & Service Centre based in Mumbai, underline the importance of the CPHI & PMEC and explain how machine manufacturers are meeting the requirements of pharma companies in India

Sigachi isn't just a supplier; we are a strategic partner
Amit Raj Sinha, MD and CEO, Sigachi explains that his company's strategic growth and diversification across excipients, APIs, food, nutrition, and personal care are driven by innovation, sustainability, and a customer-first approach. The company's focus on quality, regulatory compliance, and digital transformation positions it as a trusted global partner across industries

Driving pharma efficiency with Körber's Ecosystem solutions
Körber streamlines pharmaceutical manufacturing with a comprehensive ecosystem of advanced and connected solutions. Their guiding principle is simple: Everything, effortlessly, in one place. With top-tier solutions spanning cutting-edge machinery, packaging, materials, software, and expert guidance, Körber propels clients towards establishing their Factory of Excellence, optimizing every process for efficiency and safety, inform Rajesh Vedak President Körber India, Körber Business Area Pharma.

Customer satisfaction and innovation drive JULABO's growth in global temperature control markets
Markus Juchheim, Managing Director of JULABO GmbH, shares insights with Express Pharma on the company's strategies for innovation, regulatory compliance, and customer-focused growth in the evolving temperature control technology market.

From lab to plant: Process safety data sheets as the key to safe scale-up
Mario Toubes-Rodrigo, Global Applications Leader, H.E.L Group highlights that process safety testing is essential in API manufacturing to safely scale production from lab to industrial levels. Advanced techniques help identify and mitigate hazards, ensuring safe, compliant, and efficient processes

Eco-conscious packaging: A strategic opportunity
Larger pharma companies are applying sustainable practices and eco-conscious materials to packaging and labelling, leading to positive environmental outcomes, increasing operational efficiency and improving consumer perception. But wooing environmentally-conscious consumers often needs a sizeable capex, which could be a hurdle for pharma MSMEs. Viveka Roychowdhury reports that MSMEs can maximise this strategic opportunity once they identify areas which can improve efficiency through optimisation and harmonisation, at reasonable costs

ACG covers every facet of the pharma process, all under one roof and seamlessly interconnected
ACG Inspection's Life Sciences Cloud will be making its India debut at the CPhI India- PMec India 2024 show. Udit Singh, CEO, ACG Inspection spoke to Viveka Roychowdhury on its USPs, highlighting its modular nature, the zero cost of transition from existing systems, and the advantage of ACG's long term partnerships with global standard setting organisations like GS1 which allow its platforms to deliver regulatory compliance deliverables in advance

We, at OmniActive, invest over 10 per cent of the total outlay into R&D
Sanjaya Mariwala, Executive Chairman and MD, OmniActive Health Technologies shares insights into the company's significant investments in R&D, advanced testing capabilities, and strategies for optimising bioactive ingredients. He also discusses the impact of regulatory changes, quality assurance, and consumer education in the evolving nutraceutical landscape, in an interview with Viveka Roychowdhury
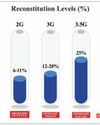
Save coating time, boost production efficiency
Instacoat 4G unique formulation includes an optimal ratio of polymer, plasticizer, and pigments, ensuring a smooth, uniform coating while significantly reducing preparation and process time

Pharma companies can optimise R&D budgets using advanced technologies
Pharma companies can optimise their R&D budgets to maximise innovation and deliver high-value therapies by using combination of new technologies to enhance innovation, feed the drug-development pipeline and speed to market.
The wide-ranging health benefits of Pycnogenol for women of all ages
Dr Franziska Weichmann, Manager of Scientific Communications and Product Development at Horphag Research, highlights benefits of Pycnogenol, a French maritime pine bark extract, offering women across all life stages a natural way to manage the physical and emotional challenges tied to hormonal changes

Indian pharma industry: Urgent need for action in the manufacturing sector
Dr Ajay Babu Pazhayattil, President of cGMPWorld, offers invaluable insights from FDA Form 483 observations issued to Indian pharma facilities, shedding light on the industry's critical challenges today. His analysis provides immediate and practical plans to minimise regulatory compliance risks. These measures will help reinforce the Indian industry's commitment to supplying the much-needed lifesaving drug products for the regulated US market

Nutraceutical M&As: How strategic mergers are shaping the health and wellness market
The nutraceutical industry has seen a surge in M&A activity over the past three years as companies pursue strategic acquisitions to strengthen market positions, diversify offerings, and drive innovation. Sudhindran N, GM - Corporate Affairs, Nutrify Today explores key trends, motivations, notable deals, and future growth prospects shaping the industry