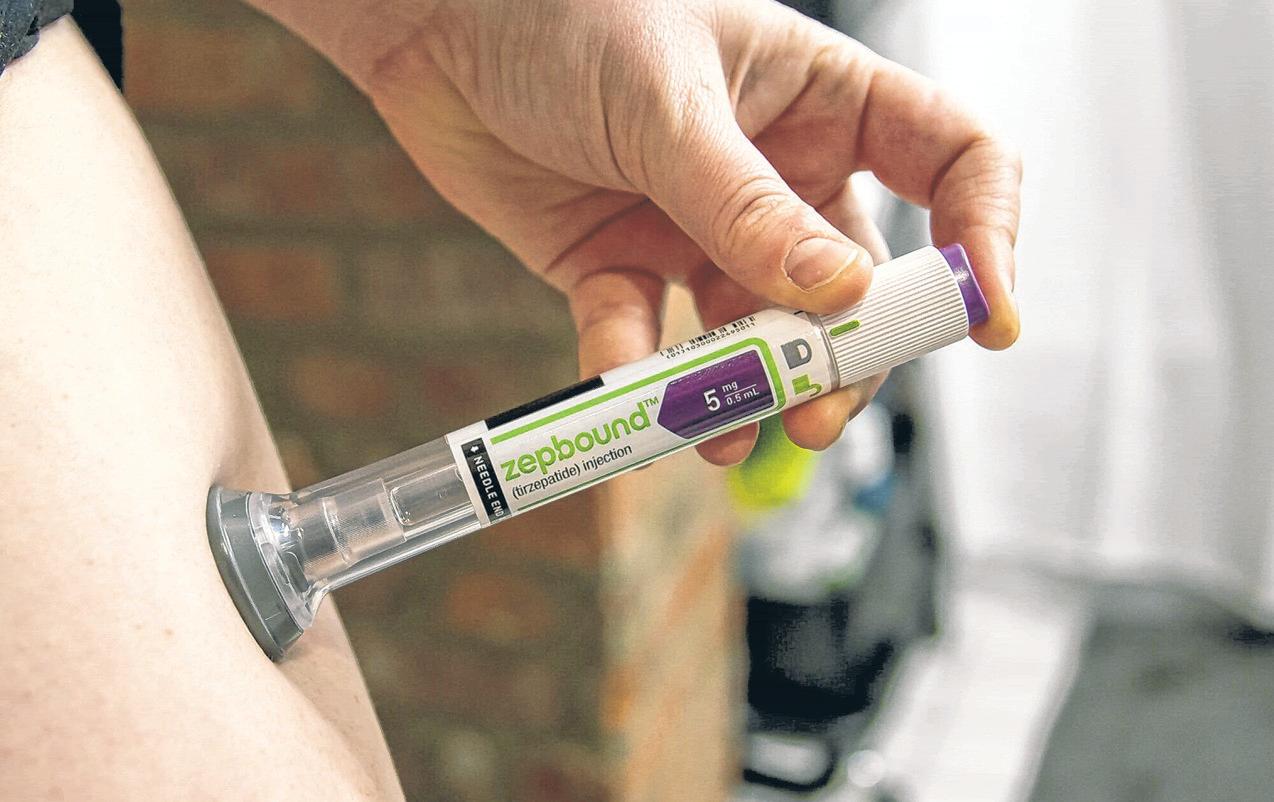
Eli Lilly said the Food and Drug Administration approved Zepbound for moderate-to-severe obstructive sleep apnea in adults with obesity.
The company said Zepbound, or tirzepatide, is the first and only prescription medicine for adults suffering from this condition. The drug was launched in the U.S. for the treatment of adults with obesity or who are overweight with weight-related comorbidities in November 2023.
Esta historia es de la edición December 23, 2024 de The Wall Street Journal.
Comience su prueba gratuita de Magzter GOLD de 7 días para acceder a miles de historias premium seleccionadas y a más de 9,000 revistas y periódicos.
Ya eres suscriptor ? Conectar
Esta historia es de la edición December 23, 2024 de The Wall Street Journal.
Comience su prueba gratuita de Magzter GOLD de 7 días para acceder a miles de historias premium seleccionadas y a más de 9,000 revistas y periódicos.
Ya eres suscriptor? Conectar