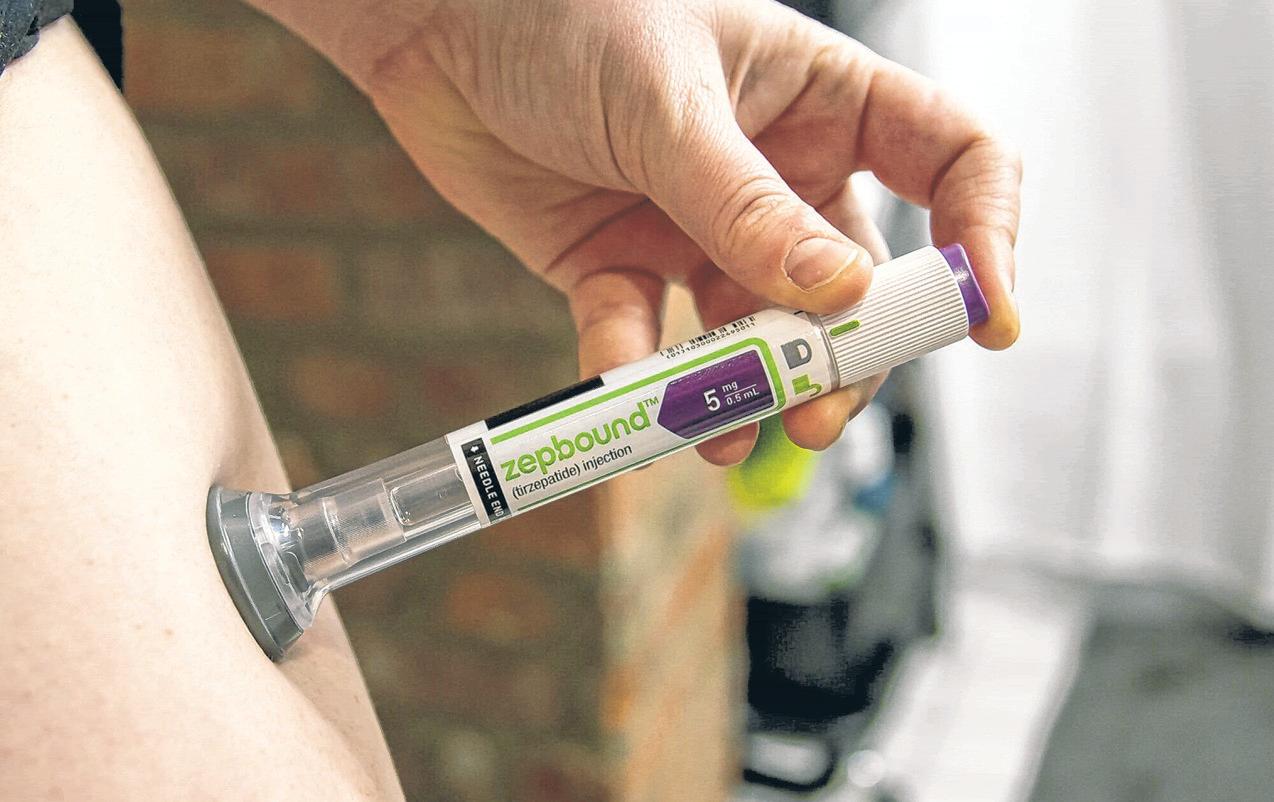
Eli Lilly said the Food and Drug Administration approved Zepbound for moderate-to-severe obstructive sleep apnea in adults with obesity.
The company said Zepbound, or tirzepatide, is the first and only prescription medicine for adults suffering from this condition. The drug was launched in the U.S. for the treatment of adults with obesity or who are overweight with weight-related comorbidities in November 2023.
Denne historien er fra December 23, 2024-utgaven av The Wall Street Journal.
Start din 7-dagers gratis prøveperiode på Magzter GOLD for å få tilgang til tusenvis av utvalgte premiumhistorier og 9000+ magasiner og aviser.
Allerede abonnent ? Logg på
Denne historien er fra December 23, 2024-utgaven av The Wall Street Journal.
Start din 7-dagers gratis prøveperiode på Magzter GOLD for å få tilgang til tusenvis av utvalgte premiumhistorier og 9000+ magasiner og aviser.
Allerede abonnent? Logg på