
On September 20, theWorld Health Organisation endorsed a protocol for testing African herbal medicine as potential treatments for the coronavirus which causes COVID-19 as well as other epidemics. While India's AYUSH ministry must be feeling vindicated that they had approved clinical research studies on four Ayurvedic formulations way back on May 22, it is important to note that the WHO stressed that phase III clinical trials are pivotal in fully assessing the safety and efficacy of a new medical product.
The COVID-19 pandemic has seen an unprecedented interest in products with a ‘natural’ tag, as people try to undo years of unhealthy eating habits, lack of exercise and stressful work-life imbalances. While big FMCG companies such as Dabur reportedly clocked a 400 per cent surge in demand for Dabur Chyawanprash and an 80 per cent traction in Dabur Honey in the early months of the lockdown, the rising tide has lifted many newer companies and startups in the natural ingredients space. For instance, Mumbai-based OZiva reported a 40 per cent increase in queries especially in categories related to immunity and everyday fitness while Upakarma Ayurveda clocked a 35 per cent surge in the same categories.
But with so many brands crowding the natural space, and more joining in the gold rush triggered by the COVID-19 pandemic, how are both newbies and established brands standing out? Or will some brands lose out in the stampede? And how can they take on the cost versus benefits argument, especially when they seem to lack hard evidence and data that they are actually beneficial to users as they have not gone through the same regulatory approval process as prescription medicines? Here are some strategies at play.
Getting evidence on their side
This story is from the October 2020 edition of Express Pharma.
Start your 7-day Magzter GOLD free trial to access thousands of curated premium stories, and 9,000+ magazines and newspapers.
Already a subscriber ? Sign In
This story is from the October 2020 edition of Express Pharma.
Start your 7-day Magzter GOLD free trial to access thousands of curated premium stories, and 9,000+ magazines and newspapers.
Already a subscriber? Sign In

Driving pharma efficiency with Körber's Ecosystem solutions
Körber streamlines pharmaceutical manufacturing with a comprehensive ecosystem of advanced and connected solutions. Their guiding principle is simple: Everything, effortlessly, in one place. With top-tier solutions spanning cutting-edge machinery, packaging, materials, software, and expert guidance, Körber propels clients towards establishing their Factory of Excellence, optimizing every process for efficiency and safety, inform Rajesh Vedak President Körber India, Körber Business Area Pharma.

Customer satisfaction and innovation drive JULABO's growth in global temperature control markets
Markus Juchheim, Managing Director of JULABO GmbH, shares insights with Express Pharma on the company's strategies for innovation, regulatory compliance, and customer-focused growth in the evolving temperature control technology market.

From lab to plant: Process safety data sheets as the key to safe scale-up
Mario Toubes-Rodrigo, Global Applications Leader, H.E.L Group highlights that process safety testing is essential in API manufacturing to safely scale production from lab to industrial levels. Advanced techniques help identify and mitigate hazards, ensuring safe, compliant, and efficient processes

Eco-conscious packaging: A strategic opportunity
Larger pharma companies are applying sustainable practices and eco-conscious materials to packaging and labelling, leading to positive environmental outcomes, increasing operational efficiency and improving consumer perception. But wooing environmentally-conscious consumers often needs a sizeable capex, which could be a hurdle for pharma MSMEs. Viveka Roychowdhury reports that MSMEs can maximise this strategic opportunity once they identify areas which can improve efficiency through optimisation and harmonisation, at reasonable costs

ACG covers every facet of the pharma process, all under one roof and seamlessly interconnected
ACG Inspection's Life Sciences Cloud will be making its India debut at the CPhI India- PMec India 2024 show. Udit Singh, CEO, ACG Inspection spoke to Viveka Roychowdhury on its USPs, highlighting its modular nature, the zero cost of transition from existing systems, and the advantage of ACG's long term partnerships with global standard setting organisations like GS1 which allow its platforms to deliver regulatory compliance deliverables in advance

We, at OmniActive, invest over 10 per cent of the total outlay into R&D
Sanjaya Mariwala, Executive Chairman and MD, OmniActive Health Technologies shares insights into the company's significant investments in R&D, advanced testing capabilities, and strategies for optimising bioactive ingredients. He also discusses the impact of regulatory changes, quality assurance, and consumer education in the evolving nutraceutical landscape, in an interview with Viveka Roychowdhury
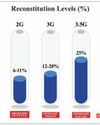
Save coating time, boost production efficiency
Instacoat 4G unique formulation includes an optimal ratio of polymer, plasticizer, and pigments, ensuring a smooth, uniform coating while significantly reducing preparation and process time

Pharma companies can optimise R&D budgets using advanced technologies
Pharma companies can optimise their R&D budgets to maximise innovation and deliver high-value therapies by using combination of new technologies to enhance innovation, feed the drug-development pipeline and speed to market.
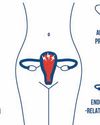
The wide-ranging health benefits of Pycnogenol for women of all ages
Dr Franziska Weichmann, Manager of Scientific Communications and Product Development at Horphag Research, highlights benefits of Pycnogenol, a French maritime pine bark extract, offering women across all life stages a natural way to manage the physical and emotional challenges tied to hormonal changes

Indian pharma industry: Urgent need for action in the manufacturing sector
Dr Ajay Babu Pazhayattil, President of cGMPWorld, offers invaluable insights from FDA Form 483 observations issued to Indian pharma facilities, shedding light on the industry's critical challenges today. His analysis provides immediate and practical plans to minimise regulatory compliance risks. These measures will help reinforce the Indian industry's commitment to supplying the much-needed lifesaving drug products for the regulated US market