The new Medical Devices Rules, 2017 seek to remove regulatory bottlenecks to make in India, facilitate ease of doing business while ensuring availability of better medical devices for patient care and safety. The rules will also regulate a much larger set of medical devices under a framework customized for medical devices. The stakeholders are hoping that the system will be industry friendly and will indeed create an ecosystem that will enable to boost medical technology innovation and manufacturing in the country.

On January 31, 2017 the Ministry of Health and Family Welfare has notified Medical Devices Rules, 2017. The new Rules have been framed in conformity with Global Harmonisation Task Force (GHTF) framework and conform to best international practices. The rules will come in to force with effect from January 1, 2018. Only 15 categories of medical devices are, at present, regulated as drugs and to that extent, the current regulatory practices in India were not fully geared to meet the requirements of medical devices sector in the country.
Medical devices will, under the new Rules, be classified as per GHTF practice, based on associated risks, into Class A (low risk), Class B (low moderate risk), Class C (moderate high risk) and Class D (high risk). The manufacturers of medical devices will be required to meet risk proportionate regulatory requirements that have been specified in the Rules and are based on best international practices.
With a view to bring in the highest degree of professionalism in regulation of medical devices, a system of ‘Third Party Conformity Assessment and Certification’ through Notified Bodies is envisaged. The Notified Bodies will be accredited by the National Accreditation Board for Certification Bodies (NABCB). The NABCB will, before accrediting Notified Bodies, assess their competence in terms of required human resources and other requirements. These Bodies will undertake verification and assessment of Quality Management System of Medical Device Manufacturers of Class A and Class B category and may, on as required basis, be called upon to render assistance for regulation of Class C and D medical devices also.
Bu hikaye Bio Spectrum dergisinin November 2017 sayısından alınmıştır.
Start your 7-day Magzter GOLD free trial to access thousands of curated premium stories, and 9,000+ magazines and newspapers.
Already a subscriber ? Giriş Yap
Bu hikaye Bio Spectrum dergisinin November 2017 sayısından alınmıştır.
Start your 7-day Magzter GOLD free trial to access thousands of curated premium stories, and 9,000+ magazines and newspapers.
Already a subscriber? Giriş Yap

Sartorius Stedim Biotech opens new centre for bioprocess innovation in US
Sartorius Stedim Biotech, a leading partner of the biopharmaceutical industry, has opened its new centre for Bioprocess Innovation in Marlborough, Massachusetts in the US.

Agilent introduces innovative Mito-rOCR assay kit for mitochondrial research
Agilent Technologies Inc. has announced the new MitorOCR Assay Kit.

Qiagen launches AI-extension of Ingenuity Pathway Analysis for automatic interpretation of biological data
Qiagen has announced the launch of Ingenuity Pathway Analysis (IPA) Interpret, a new feature designed to simplify and accelerate the interpretation of complex biological data.

Parse Biosciences announces expansion of Evercode BCR product line
Parse Biosciences, a leader in scalable single cell sequencing solutions, has announced the expansion of its Evercode BCR product line to enable applications in mice.
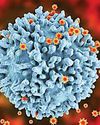
New technology for unambiguous detection of HIV genome using tailored fluorogenic tests
A team of scientists at Jawaharlal Nehru Centre for Advanced Scientific Research (JNCASR), has developed a novel diagnostic technology known as the GQ Topology-Targeted Reliable Conformational Polymorphism (GQ-RCP) platform.

Gene mutation likely cause for developing autism in early childhood: RGCB study
Autism, a developmental disorder that causes functional abnormalities in brain development, is caused by a combination of environmental and genetic factors with its symptoms manifesting in childhood as early as the age of two years.

IIIT-Delhi unveils AI platform for discovering molecules that promote healthy ageing
A team of researchers from Indraprastha Institute of Information Technology Delhi (IIIT-Delhi) has developed AgeXtend, a groundbreaking artificial intelligence (AI)-based platform that is set to transform the search for molecules promoting healthy ageing.

RSSDI study lays focus on Yoga and Diabetes Prevention
Dr Jitendra Singh, Union Minister of State (Independent Charge) for Science and Technology, recently released the landmark study by Research Society for Study of Diabetes in India (RSSDI) on 'Yoga and Diabetes Prevention'.

Dr Alok Khullar steps in as Group CEO of RJ Corp Healthcare
RJ Corp Healthcare, a leading name in the healthcare sector through its renowned entities Cocoon Hospital and Cryoviva Stem Cell Bank, has announced the appointment of Dr Alok Khullar as Group Chief Executive Officer (CEO).

Entod Pharmaceuticals on-boards Dr Anish Desai as Scientific & Research adviser
Mumbai-based Entod Pharmaceuticals has appointed Dr Anish Desai as its Scientific and Research Adviser.