
In Europe, the European Medicines Agency estimates that up to 36 million people are diagnosed with an RD. However, approximately 5 per cent of RDs have US FDAapproved treatment options, while up to 15 per cent have at least one drug that exhibits potential for treatment, diagnosis, or prevention. The growing number of unaddressed RD needs is a major catalyst for R&D. There is a need for novel medicine to treat RDs that currently have limited therapeutic choices. Let's explore further.
Governments across the world have regulatory incentives to support orphan drug (OD) development. It is more appealing for pharmaceutical firms to engage in R&D for RDS because of these incentives, which include prolonged exclusivity, tax benefits, and simplified and expedited regulatory procedures and approvals.
Recent advancements in precision medicine and informatics, such as big data analytics, multi-omics, nanomedicine, gene-editing techniques, and nextgeneration diagnostics, have created opportunities to develop specific and individualised therapies for RDs. The convergence of cancer and RDs is becoming evident. Precision oncology and tailored medicine for rare tumours are emerging as prominent themes in the discipline, facilitating the OD industry's expansion.
There are multiple challenges restraining the development and adoption of orphan drugs. Owing to low awareness of RDs, many patients go undetected for extended periods. Apart from diagnosis, both prognostics and therapy are seeing significant gaps that must be filled. Challenges in prognosis assessment are due to the absence of reliable parameters to measure improvement and/or biomarkers as well as a lack of knowledge of underlying pathophysiological pathways.
Furthermore, a limited patient sample size prevents the derivation of statistically significant parameters.
Bu hikaye Bio Spectrum dergisinin August 2024 sayısından alınmıştır.
Start your 7-day Magzter GOLD free trial to access thousands of curated premium stories, and 9,000+ magazines and newspapers.
Already a subscriber ? Giriş Yap
Bu hikaye Bio Spectrum dergisinin August 2024 sayısından alınmıştır.
Start your 7-day Magzter GOLD free trial to access thousands of curated premium stories, and 9,000+ magazines and newspapers.
Already a subscriber? Giriş Yap

Sartorius Stedim Biotech opens new centre for bioprocess innovation in US
Sartorius Stedim Biotech, a leading partner of the biopharmaceutical industry, has opened its new centre for Bioprocess Innovation in Marlborough, Massachusetts in the US.

Agilent introduces innovative Mito-rOCR assay kit for mitochondrial research
Agilent Technologies Inc. has announced the new MitorOCR Assay Kit.

Qiagen launches AI-extension of Ingenuity Pathway Analysis for automatic interpretation of biological data
Qiagen has announced the launch of Ingenuity Pathway Analysis (IPA) Interpret, a new feature designed to simplify and accelerate the interpretation of complex biological data.

Parse Biosciences announces expansion of Evercode BCR product line
Parse Biosciences, a leader in scalable single cell sequencing solutions, has announced the expansion of its Evercode BCR product line to enable applications in mice.
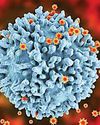
New technology for unambiguous detection of HIV genome using tailored fluorogenic tests
A team of scientists at Jawaharlal Nehru Centre for Advanced Scientific Research (JNCASR), has developed a novel diagnostic technology known as the GQ Topology-Targeted Reliable Conformational Polymorphism (GQ-RCP) platform.

Gene mutation likely cause for developing autism in early childhood: RGCB study
Autism, a developmental disorder that causes functional abnormalities in brain development, is caused by a combination of environmental and genetic factors with its symptoms manifesting in childhood as early as the age of two years.

IIIT-Delhi unveils AI platform for discovering molecules that promote healthy ageing
A team of researchers from Indraprastha Institute of Information Technology Delhi (IIIT-Delhi) has developed AgeXtend, a groundbreaking artificial intelligence (AI)-based platform that is set to transform the search for molecules promoting healthy ageing.

RSSDI study lays focus on Yoga and Diabetes Prevention
Dr Jitendra Singh, Union Minister of State (Independent Charge) for Science and Technology, recently released the landmark study by Research Society for Study of Diabetes in India (RSSDI) on 'Yoga and Diabetes Prevention'.

Dr Alok Khullar steps in as Group CEO of RJ Corp Healthcare
RJ Corp Healthcare, a leading name in the healthcare sector through its renowned entities Cocoon Hospital and Cryoviva Stem Cell Bank, has announced the appointment of Dr Alok Khullar as Group Chief Executive Officer (CEO).

Entod Pharmaceuticals on-boards Dr Anish Desai as Scientific & Research adviser
Mumbai-based Entod Pharmaceuticals has appointed Dr Anish Desai as its Scientific and Research Adviser.