CATEGORIES

Customer satisfaction and innovation drive JULABO's growth in global temperature control markets
Markus Juchheim, Managing Director of JULABO GmbH, shares insights with Express Pharma on the company's strategies for innovation, regulatory compliance, and customer-focused growth in the evolving temperature control technology market.

From lab to plant: Process safety data sheets as the key to safe scale-up
Mario Toubes-Rodrigo, Global Applications Leader, H.E.L Group highlights that process safety testing is essential in API manufacturing to safely scale production from lab to industrial levels. Advanced techniques help identify and mitigate hazards, ensuring safe, compliant, and efficient processes

Eco-conscious packaging: A strategic opportunity
Larger pharma companies are applying sustainable practices and eco-conscious materials to packaging and labelling, leading to positive environmental outcomes, increasing operational efficiency and improving consumer perception. But wooing environmentally-conscious consumers often needs a sizeable capex, which could be a hurdle for pharma MSMEs. Viveka Roychowdhury reports that MSMEs can maximise this strategic opportunity once they identify areas which can improve efficiency through optimisation and harmonisation, at reasonable costs

ACG covers every facet of the pharma process, all under one roof and seamlessly interconnected
ACG Inspection's Life Sciences Cloud will be making its India debut at the CPhI India- PMec India 2024 show. Udit Singh, CEO, ACG Inspection spoke to Viveka Roychowdhury on its USPs, highlighting its modular nature, the zero cost of transition from existing systems, and the advantage of ACG's long term partnerships with global standard setting organisations like GS1 which allow its platforms to deliver regulatory compliance deliverables in advance

We, at OmniActive, invest over 10 per cent of the total outlay into R&D
Sanjaya Mariwala, Executive Chairman and MD, OmniActive Health Technologies shares insights into the company's significant investments in R&D, advanced testing capabilities, and strategies for optimising bioactive ingredients. He also discusses the impact of regulatory changes, quality assurance, and consumer education in the evolving nutraceutical landscape, in an interview with Viveka Roychowdhury
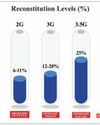
Save coating time, boost production efficiency
Instacoat 4G unique formulation includes an optimal ratio of polymer, plasticizer, and pigments, ensuring a smooth, uniform coating while significantly reducing preparation and process time

Pharma companies can optimise R&D budgets using advanced technologies
Pharma companies can optimise their R&D budgets to maximise innovation and deliver high-value therapies by using combination of new technologies to enhance innovation, feed the drug-development pipeline and speed to market.
The wide-ranging health benefits of Pycnogenol for women of all ages
Dr Franziska Weichmann, Manager of Scientific Communications and Product Development at Horphag Research, highlights benefits of Pycnogenol, a French maritime pine bark extract, offering women across all life stages a natural way to manage the physical and emotional challenges tied to hormonal changes

Indian pharma industry: Urgent need for action in the manufacturing sector
Dr Ajay Babu Pazhayattil, President of cGMPWorld, offers invaluable insights from FDA Form 483 observations issued to Indian pharma facilities, shedding light on the industry's critical challenges today. His analysis provides immediate and practical plans to minimise regulatory compliance risks. These measures will help reinforce the Indian industry's commitment to supplying the much-needed lifesaving drug products for the regulated US market

Nutraceutical M&As: How strategic mergers are shaping the health and wellness market
The nutraceutical industry has seen a surge in M&A activity over the past three years as companies pursue strategic acquisitions to strengthen market positions, diversify offerings, and drive innovation. Sudhindran N, GM - Corporate Affairs, Nutrify Today explores key trends, motivations, notable deals, and future growth prospects shaping the industry

Rising research costs, complex drug systems, stringent regulations, and global pressures pose major threats to pharma R&D
According to one of the published reports, the pharma industry invested about $83 billion in R&D in 2019, which is about 10 times the research and development (R&D) spent per year in the 1980s, after adjusting for the effects of inflation.

Harnessing data analytics to propel drug discovery and development in 2025
As the pharma landscape evolves, data analytics will be at the forefront of drug discovery and development, transforming traditional methods. By leveraging data-driven insights, companies can expedite R&D, optimise clinical trials, and personalise treatments, bringing life-saving therapies to market more efficiently, explains Biju Davis, SVP, Engineering, Model N

Strengthening our presence within APAC through increased penetration is a key focus
Aanchal Tomar, Executive Director, Asia Pacific, Lonza Capsules Health Ingredients speaks about Lonza Capsules Health Ingredients’ strategic growth, challenges, and sustainability initiatives in the APAC market, showcasing their innovative capsule solutions and health ingredients that cater to the region's diverse pharmaceutical and nutraceutical needs, in an interview with Express Pharma

Responsible Sourcing and Manufacturing: Growing towards a sustainable future with Lonza
In response to the establishment of environmental impact targets by the pharmaceutical industry, Lonza promotes responsible sourcing practices, sustainable manufacturing standards, and renewable energy utilisation showcasing that responsible manufacturing can assist our customers to reach their sustainability goals, highlights Christine Lebeault, Head of Applied Sustainability, Lonza Capsules and Health Ingredients

Rethinking R&D to balance efficiency, innovation and sustainability
In an era of skyrocketing research costs and complex drug delivery challenges, pharma industry leaders share their insights on adopting advanced technologies, data-driven decision-making, and sustainable practices to optimise R&D and drive impactful therapies to market

Powerful Process Control with IND500x Weighing Indicators
Ensuring consistent quality in hazardous environments, particularly in Ex-Areas (Zone 1/21, Division 1), is a critical aspect of industrial operations. The IND500x weighing indicators from METTLER TOLEDO provide a robust solution for these challenging settings, offering optimized safety and productivity.

Ensuring clean room integrity with prime clean reset high-speed doors
Prime Clean Reset high-speed doors ensure airtight seals, minimising air permeability and contamination in clean rooms. Designed for sensitive environments, they enhance operational efficiency and meet rigorous regulatory standards, making them ideal for pharmaceutical and biotech industries

Complete environmental monitoring solution - testo Saveris Pharma
There are several critical applications in the industry like research and development that demand for continuous & reliable monitoring of important environmental parameters.

Adaptive Manufacturing:-The new flexibility in medical device assembly
Unlike traditional, rigid production lines, B&R's adaptive machines seamlessly adjust to the requirements of each device, dynamically adapting to each unique process

Reshaping pharma safety with active packaging innovations
CILICANT is revolutionising active packaging with solutions tailored for the most sensitive formulations. Meet the innovators driving stability, safety, and impurity control in pharma packaging at CPhI India

UNLOCKING GROWTH FRONTIERS
Vadodara Pharma Summit 2024 brought together industry leaders and experts to highlight the city's pivotal role in India's pharma landscape and explore collaborative strategies to strengthen India's pharma innovation ecosystem

Optimising cloud provisioning for pharmaceutical compliance and operational excellence
Suresh Perikala, Senior Engineering Lead - DevOps and Cloud Engineering Practice, Altimetrik

Accelerating pharma's sustainability efforts
Avinaw Prasad, Director, Climate Change & Sustainability, Deloitte India explains why integrating sustainability into the core pharma business strategy is essential for long-term success, analyses key focus areas and policy pushes which can incentivise companies to further evolve on the sustainability path

Drug Repurposing: Unlocking access to rare solutions
Drug repurposing, a quick, cost-effective path to deliver existing treatments to patients, can serve to expedite and expand healthcare access for patients with rare diseases

Optimising spend, maximising growth
Express Pharma, in partnership with IPA and CHEMEXCIL, powered by SAP, recently organised a thought leadership conclave on 'Reimagining spend management and supply chain' where cross functional experts explored strategies to drive value through supply chain transformation

Green manufacturing in pharma: Sustainable oligonucleotide production on an industrial scale
Philipp Markolin, Scientific Marketing Manager at peptide and oligonucleotide manufacturers Bachem, shares their expertise on sustainable production methods in the pharma industry

Data and digital is a force to reckon with when it comes to India
In a recent interview, Suneela Thatte, VP and Head - Healthcare R&D for India, Merck KGaA Darmstadt discussed the evolution and strategic significance of the company's Global Capability Center (GCC) in India, established in 2020. She highlighted that while the GCC was initially set up for cost savings in drug development, the focus has shifted to harnessing India's vast talent pool, particularly in the scientific and medical fields, in an interview with Viveka Roychowdhury

Towards a healthier, more equitable and innovative Indian healthcare sector
Rishabh Bindlish, MD & Partner, Co-leads Boston Consulting Group Healthcare practice in India and Ayushi Shukla, Senior Knowledge Analyst, BCG highlight that the government needs to pave the way for a healthier, more equitable future with strategic investments and policy reforms

Patient centricity: How is the pharma industry addressing patient reach?
Arecent report from Access to Medicine Foundation assesses current approaches adopted by some of the largest pharma companies to measure and report patient reach, mapping the existing landscape and highlighting interventions that are critical to ensure more patients benefit from increased access to medicine. Excerpts from the report

PROSOLV® SMCC 50-A high-functional excipient from JRS Pharma
The development of coprocessed, multi-functional excipients has enabled formulators to address multiple challenges with a single excipient, resulting in enhanced production and better finished product quality.